FUROSCIX USE IN THEIR COMFORT ZONE
EASE OF USE
FUROSCIX IS DESIGNED FOR SUBCUTANEOUS ADMINISTRATION AT HOME BY THE PATIENT OR CAREGIVER1,2
- FUROSCIX utilizes a pre-programmed, single-use On-Body Infusor with pre-filled cartridge1
- Once attached, the On-body Infusor is activated with the press of a single button2
For more information on how to use FUROSCIX, view our application video.
Watch NowTHE ON-BODY INFUSOR AND INSTRUCTIONS FOR USE WERE UNDERSTOOD AND EFFECTIVELY USED BY THE INTENDED POPULATIONS*2


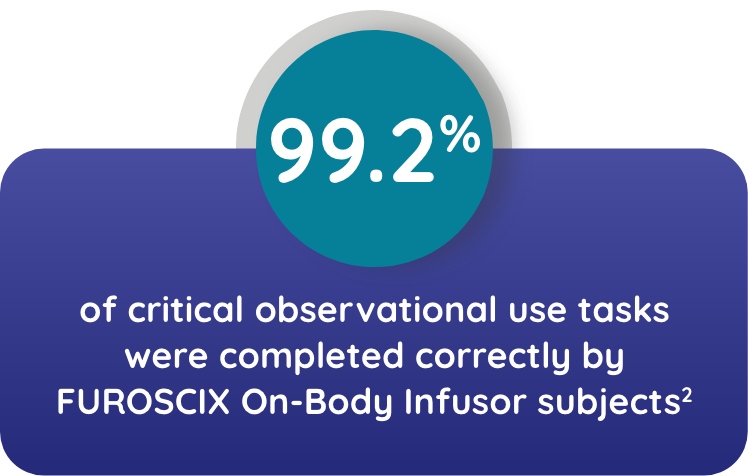
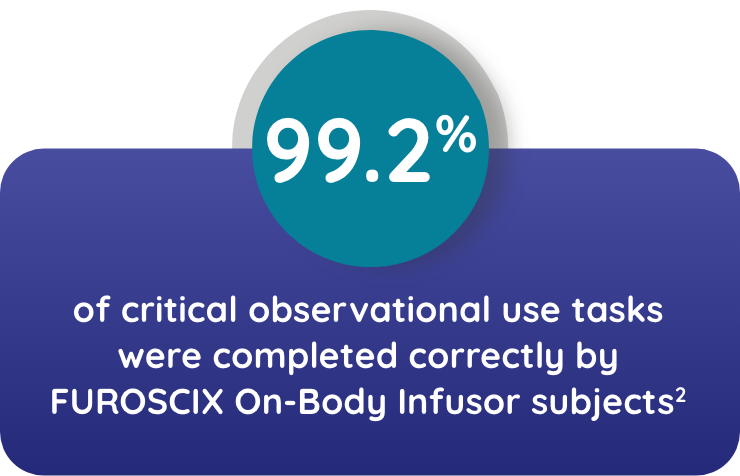



*Trained participants attended an HCP-conducted walk-through of the instructions for use. Participants performed all tasks the following day. Patients were representative of the intended user population in terms of age, gender, ethnicity, and education.
Tolerability
SYSTEMIC ADVERSE REACTIONS WITH FUROSCIX WERE CONSISTENT WITH THOSE REPORTED FROM PATIENTS ADMINISTERED IV FUROSEMIDE1,3
The most common adverse reactions with the FUROSCIX On-Body Infusor were erythema, bruising, edema, and infusion site pain.1
THE NUMBER OF DOSES OF FUROSCIX SHOULD BE INDIVIDUALIZED BY THE HCP BASED ON THE PATIENT’S THERAPEUTIC RESPONSE
- FUROSCIX is not for chronic use and should be replaced with oral diuretics as soon as practical1
- Monitoring of patients taking FUROSCIX is consistent with diuretic guidance4,5
FUROSCIX has helpful resources for you and your patients.
Learn MoreReferences: 1. FUROSCIX [prescribing information]. Burlington, MA: scPharmaceuticals Inc.; 2022. 2. Andre AD, Mohr J, Cornelius B, et al. 2020. Successful validation of a wearable, on-body infusor for subcutaneous administration of Furoscix® in heart failure patients, caregivers, and health care practitioners. [Poster]. The HFSA Virtual Annual Scientific Meeting, September 30 – October 6, 2020. 3. Furosemide Injection, USP [package insert]. Lake Forest, IL: Hospira, Inc.; 2022. 4. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi: 10.1161/CIR.0000000000001063. 5. Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137-155. doi:10.1002/ejhf.1369.